- Next to T. B. Hospital Main Gate, Koch’s House, Jerbai Wadia Road, Sewri
TARGETED IMAGING AND THERAPY
PROSTATE CANCER
WHY CHOOSE 68 GA PSMA FOR PROSTATE CANCER STAGING ?
All relevant informaon in 1 scan!
PSMA PET-CT can be used to image and characterize the primary tumor itself, lymph nodes (independent of size), distal visceral metastases and the bone metastases, all in 1 single scan.
You don’t miss any lesion!
Superior tumour to background contrast compared to other molecular tracers allows for detection of disease in small regional nodes and distant disease in bones or visceral organs.
It has the highest sensitivity for early detect on of site of recurrence in patients with scans showing disease focus in Sr. PSA levels as low as 0.2 ng.dl.
Concurrent diagnostic contrast-enhanced CT scan of the chest, abdomen and pelvis allows anatomical correlation to focus of abnormal PSMA uptake, significantly increasing sensitivity and specificity of the examination.
You don’t pay more !
Similar cost to patients when compared with combined 18F-NaF or 99M-TC MDP bone scan and diagnostic CT of the chest, abdomen and pelvis.
Potential indications for 68Ga-PSMA ligand PET/CT
Primary staging in intermediate or high-risk disease according to D’Amico classification Biochemical recurrence with low PSA-values (0.2 ng/ml – l ng/ml)
Biopsy targeting after previous negative biopsy, but high suspicion of prostate cancer monitoring of systemic treatment.
Active surveillance Treatment monitoring in metastatic castration- resistant PC undergoing radioligand therapy targeting PSMA (e.g. 177Lu-PSMA-ligand)
PSMA-PET-CT WITH COREGISTERED MRI:
Primary staging in intermediate or high-risk disease according to D’Amico classification Biochemical recurrence with low PSA-values (0.2 ng/ml – I ng/ml)
Biopsy targeting after previous negative biopsy, but high suspicion of prostate cancer monitoring of systemic treatment.
Active surveillance Treatment monitoring in metastatic castration- resistant PC undergoing radioligand therapy targeting PSMA(e.g. 177Lu-PSMA-ligand)
RADIOUGAND THERAPY FOR PROSTATE CANCERS WITH LUTETIUM-PSMA:
Radioligand therapy has very promising results in castrate resistant metastatic prostate cancers.
2 types of radionuclide therapies are available for metastatic prostate cancer:
Beta emitter Lutetium-PSMA
Alpha emitter Actinium
The treatment is administered as an intravenous infusion followed by observation in radiation isolation room.
The results are very promising, with an objective response in over 60% cases resistant to all conventional hormone and chemotherapy with overall progression-free survival rate increased by 11-16 months.
NEUROENDOCRINE TUMOR
DOTANOC PET CT:
PET-CT Scan using the radiotracer 68Ga – DOTA-NOC is a highly sensitive & specific imaging technique for the detection & localization of neuroendocrine tumor.
Localization of such tumors is based on the principal of detecting somatostatin receptor, which these tumors are known to express & hence is superior to conventional imaging techniques like CT/MRI. The various roles which the imaging modality encompasses can_be summarized as under
●Detection of suspected NET
●Staging of known NET
●Restaging NET after treatment
●Monitoring response to treatment
●Selecting potential candidates for PRRT therapy & 177lu, when disease is metastasis/unresectable
Various tumors where Ga-DOTANOC PET-CT has a role are:
Well to moderately differentiated neuroendocrine tumors
Carcinoid tumors
Pheochromocytoma
Medullary carcinoma thyroid
Gastrinoma
Insulinoma
Oaddiing FDG PET provides valuable information in managing NET patients. Depending on scan findings, suitable therapy options for these patients would be PRRT (radionuclide therapy) or chemotherapy or both.
PEPTIDE RECEPTOR RADIONUCLIDE THERAPY (PRRT)
Patients having a positive Ga-DOTATATE scan with metastatic disease are potential candidates for PRRT.
PRRT uses Lutetium isotope as intravenous infusion, followed by isolation in special room.
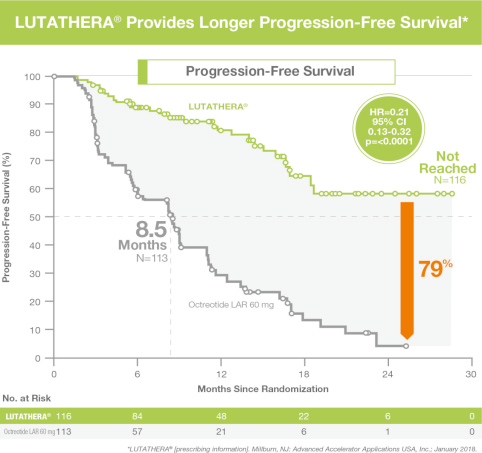
The single most important benefit of PRRT is achieving clinical results while maintaining (in fact improving) quality of life with a 79% reduction in risk of disease progression or death compared to octreotide therapy. It achieves a stable, growth-free period ranging from 1-4 years even after finishing the therapy.
PRRT is already an FDA approved therapy in the USA and has been extensively used in Europe as well.
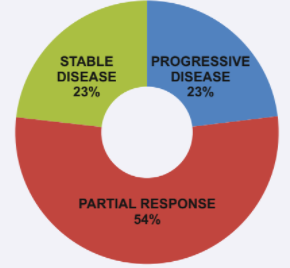
It arrests disease growth in patients presenting with progressive disease at outset in over 23% patients. More than half (54%) patients show a partial response to therapy. As per recent literature, it prolongs progression-free survival in 79% patients (when compared to treatment by cold Octreotide only).